CIENCIAS AGROPECUARIAS-Artículo Científico
PRELIMINARY EVALUATION OF PATHOGENS AFFECTING Eleodes longicollis punctigerus BLAISDELL (COLEOPTERA: TENEBRIONIDAE)
EVALUACIÓN PRELIMINAR DE PATÓGENOS QUE AFECTAN A Eleodes longicollis punctigerus BLAISDELL (COLEOPTERA: TENEBRIONIDAE)
Daniel Estiven Quiroga-Murcia1, Ingeborg Zenner de Polanía2, Francisco Javier Posada-Flórez3
1Joven Investigador Grupo de Investigación Fitosanidad, Estudiante Facultad de Ingeniería Agronómica. Universidad de Ciencias Aplicadas y Ambientales U.D.C.A, e-mail: quirogaedaniel@hotmail.com
2I.A., Ph.D., Docente-Investigador. U.D.C.A, e-mail: izenner@udca.edu.co
3 Ing. Agrónomo, Entomólogo Ph.D., Investigador Científico Independiente, e-mail: fjavierposada@hotmail.com
Rev. U.D.C.A Act. & Dic. Cient. 19(1): 37-43, Enero-Junio, 2016
SUMMARY
The false wire-worm Eleodes longicollis punctigerus, despite of been registered more than 30 years ago, is until now considered as an emerging pest in some areas of the Sabana de Bogotá, in Colombia. Therefore, within an integrated management program, the use of biological control agents have to be considered.Under field conditions, larvae and adult wireworms were found to be affected by pathogenic microorganisms, such as Paecilomyces sp., Beauveria bassiana and Metarhizium anisopliae and nematodes. Preliminary studies to determine their pathogenicity in the laboratory were undertaken, showinging that none of them were highly pathogenic. An alternative explanation may be that E. longicollis may be tolerant to the infection or the spore concentration used was to low, because the highest larval mortality observed was only 20% with the treatment pos. Paecilomyces sp., at 1x107spore concentrations. Adult mortality of 22.5% with M. anisopliae at 1x107was obtained. It was also found that the insect is susceptible to nematodes. Both nematodes and fungi should be studied under other conditions, with higher concentrations, in order to be incorporated as a complement into a strategy of preventive control.
Key words: False wire-worm, micro-biologic control, fungi, nematode.
RESUMEN
El falso gusano alambre Eleodes longicollis punctigerus, a pesar de haberse detectado hace más de 30 años, apenas últimamente, se perfila como una plaga emergente en algunas áreas de la Sabana de Bogotá, Colombia. Por ello, dentro de un plan de manejo integrado, se plantea el uso del control biológico y, luego de encontrar en campo larvas y adultos afectados por microorganismos entomopatógenos, tales como Paecilomyces sp., Beauveria bassiana y Metarhizium anisopliae y por nematodos, se decidió determinar la patogenicidad de éstos en el laboratorio. Los resultados mostraron que ninguno de ellos es altamente patogénico, existiendo la posibilidad que el insecto es tolerante a la infección o que la concentración de esporas empleada fue muy baja, ya que la mortalidad larval máxima observada fue del 20%, con el tratamiento de pos. Paecylomices sp., concentración de esporas 1x107. Sobre el adulto, se observó una mortalidad del 22,5%, con el hongo M. anisopliae, a una concentración del 1x107; también, se detectó que el insecto es susceptible a nematodos. Se concluye que, tanto estos últimos como los hongos mencionados, deben ser estudiados bajo otras condiciones y en concentraciones más altas, para poderlos incorporar como complemento de una estrategia de control preventivo, a su debido tiempo antes de la siembra, y con condiciones de adecuada humedad del suelo.
Palabras clave: Falso gusano alambre, control microbiológico, hongos, nematodo.
INTRODUCTION
Biological control has been for years a tool for population management of insect crop pests, being defined by Nicholls (2008) as the use of beneficial organisms against pests that cause damage. This concept of biological control can be based on the principle that in nature all organisms possess antagonists, which either compete or eliminate them, a process being essential for life equilibrium (Hanson, 1993).
Microorganisms are components of biological control, the so called entomopathogens, including viruses, bacteria, protozoa, rickettsia, fungi and nematodes, which can be found affecting insects, establishing symbiotic relationships such as parasitism and also causing disease and death, contributing therefore to the natural regulation of insect populations (Lacey & Kaya, 2007).
Currently, many organisms are studied and used in microbiological control programs. Successful alternatives, using entomopathogenic fungi to control arthropod pests, inhabiting water and soil environments have been developed, using primarily the genera Metharhizium, Beauveria, Sporothrix, Nomuraea, Paecilomyces among others (Alves & Lopes, 2008). According to Posada & Pava-Ripoll (2010), Bacillus spp., Beauveria bassiana (Moniliales: Moniliaceae) and Metharizum anisopliae (Hypocreales: Clavicipitaceae) are the most studied fungi being mentioned in the literature, to control several insect pests. Vieira Tiago et al. (2014) also highlighted M. anisopliae as the fungus species most studied and used for control purposes, due, among other characteristics, to its persistence in the soil.
Nematodes have multiple relationships with insects; they can be parasites, affecting their reproductive ability (Kaya & Stock, 1997), but they can also enter insects and release bacteria that are responsible for the individual's death by septicemia (Rosales et al. 2009).
Both fungi and nematodes are recommended as biological control agents of insect pests in crops of economic importance; such is the case of B. bassiana for the management of the coffee berry borer (Hypothenemus hampei).This fungus is the most commonly used and widely distributed natural enemy of this pest (Posada & Pava-Ripoll, 2010).
Beauveria bassiana is also efficient against tenebrionids such as Alphitobius diaperinus in chicken houses (Steinkraus et al. 1991) and against Tribolium castaneum pest of stored grains (Pedrini et al. 2010). Metharizium anisopliae is used to control insects of several families of the Order Coleoptera, such as Curculionidae and Scarabidae, common pests of rice, citrus and sugarcane (Nicholls Estrada, 2008). It also affects wireworms of the genus Agriotes (Elateridae) (Kabaluk & Ericsson, 2007; Ericsson et al. 2007). Another species, M. brunneum has been inclusive, evaluated against ticks of the genus Ixodes (Acari: Ixodidae).
The nematode genera Steinernema and Heterorhabditis form part of the integrated management program of Premnotrypes vorax and Tecia solanivora in potato crops (Maggiorani & Gudiño, 1996). Steinernema feltiae and S. carcocapsae (Rhabditia Steinernematidae) are considered as promising tenebrionid control agents for A. diaperinus (Geden et al. 1987) and Cyanaeus angustus (Nansen et al. 2013), respectively.
The false wire-worm, recently identified as Eleodes longicollis punctigera Blaisdell, by Dr. Charles A. Triplehorn, Ohio State University, United States, based on specimens sent by Dr. Francisco Javier Posada Flórez, and previously referenced in Colombia as Eleodes omissoides Blaisdell (Coleoptera: Tenebrionidae), causes seed losses of various plant species, especially grasses and seedlings that reach germination. Larvae and adults of the pest are considered a limiting factor in the affected crops, because the attack requires replanting, and the use of insecticides that increase costs (Quiroga- Murcia & Posada-Flórez, 2013; Zenner de Polanía et al. 2014; Calkins & Kirk, 1973; Rogers et al. 1988). It is worthwhile mentioning, that E. longicollis punctigerus was recorded for the first time in Mexico and described by Blaisdell in 1935, but in this country apparently it never became a pest.
Information on the genus Eleodes is scarce and the literature refers primarily to the taxonomy and the description of new species from Mexico and the United States (Triplehorn, 2010; Triplehorn & Cifuentes, 2011; Triplehorn & Thomas, 2011); South-American data outside those from Colombia, to our knowledge, do not exist.
Eleodes longicollis punctigera receives the common name of false wireworm because of the larval resemblance to the larvae of the family Elateridae (Coleoptera) which are considered the true wire-worms. This generic name is given to the larvae of various insects of the genera Epitragus, Anaedus, Blapstinus, Lobometopon and Ulus spp. (Saunders et al. 1998), all of the family Tenebrionidae, known pests of rice, sorghum, corn, pineapple, cotton and pastures.
Previously identified as E. omissoides in Colombian publications and in the newsletter Entomological Notes and News (NNE), the insect was first recorded in the department of Boyacá in 1977 and then in Cundinamarca in 1980 attacking pea (Pisum sativum) seeds. From then on the bulletin sporadically mentions the insect in several municipalities of Cundinamarca such as Madrid, Mosquera, Bojacá and Facatativá and Tunja (Boyacá). In addition, the authors observed that the insect is susceptible to entomopathogenic fungi and nematodes. The susceptibility of E. longicollis was intended to be confirmed with this research, even though according to Pears (2009) false wire-worms do not have registered biological control agents.
This study, evaluated the potential of some naturally occurring entomopathogens, found at ''El Remanso'', research unit of the University of Applied and Environmental Sciences, U.D.C.A Bogotá, Colombia. These biocontrol agents can be part of a sustainable control that could be integrated into a management program of E. longicollis.
MATERIALS AND METHODS
Most studies, if not stated otherwise, were carried out under laboratory conditions, 18 ± 3°C, 80% R.H. at the University.
Collection, breeding and survey: The collection of adults, larvae, pupae and eggs of the pest was done at ''El Remanso'', during three sampling periods. Two-hundred adults and some larvae were hand captured. Adults were separated by sex, resulting in 100 males and 100 females, then placed in sterile soil and fed corn and wheat seeds, and cooked rice. Their unaccounted F1 larval progeny was separated and fed with wheat. Eggs obtained from 31 adults of one sample date were counted, obtaining 70 eggs, from which 58 larvae emerged. Larvae were placed in sterilized moistened soil and fed with cooked rice.
Adults were in rectangular plastic containers (22 x 14 x 8cm high), fed with corn and wheat seeds. Most adults died, observing a survival of 15 after 10 months. Many of them were found dead with signs of cannibalism; while other showed signs of fungal infection, such as a rigid appearance, absence of odor and apparent absence of fungal infection signs. Dead adults were placed in vials with moist soil to observe sporulation.
During the first month larvae affected by Metharhizum anisopliae and Beauveria bassiana and adults affected by M. anisopliae were detected; two months later spores of the fungus identified as possibly belonging to the genus Paecilomyces were observed expanding on the soil surface, proceeding from one larva. No signs or symptoms of nematode infection were detected.
Isolation and culture of entomopathogenic organisms: After sporulation, the fungi obtained from the insects were placed in moist plastic growing chambers, and introduced to a PDA (potato, dextrose agar) medium inside polystyrene incubators, to obtain the fungal growth and development. A culture of the fungus pos. Paecilomyces was conducted in PDA obtaining a very good growth.
Once pure fungal cultures were obtained the effective SDLYM medium (Kaya & Stock, 1997), was prepared. SDLYM is composed of Agar-Sabouraud, milk, egg yolk, agar, dextrose and yeast extract.
Because no nematodes were found in false wireworm larvae, they were collected from the white grub Clavipalpus ursinus at El Remanso. These larvae were placed in plastic containers with sterilized soil and moisture at field capacity. Approximately one month later, two larvae with possible symptoms of nematode infection (showing brown color) were observed. Clavipalpus ursinus larvae were introduced in modified White traps, which consist of plastic vials with a foam disc to prevent mites, from entering, that fed on the filter paper and the insect's cadavers. After obtaining nematodes, a solution of 20 JI / cm3was prepared and within the same White traps, false wire-worm larvae of diverse instars were inoculated.
Inoculation and evaluation of pathogenicity: To determine the fungal pathogenicity on E longicollis larvae, 280 plastic vials, filled with 6g of previously sterilized soil were prepared, placing one larva per vial for each fungus species. As control 40 untreated larvae were used. Fungi produced in the above mentioned medium were applied to the larvae at two concentrations. Mortality was assessed every second day during 60 days for a total of 21 evaluations; during each observation distilled water, depending on the soil moisture, and two wheat grains as larval food were added to the vial.
Treatments corresponded to the inoculation with native strains of Metarhizum, Beauveria and pos. Paecylomices at concentrations of 1x105 and 1x107, and the control which consisted of distilled water. For this purpose, spore count was performed by dilution of a concentrated solution, using a hemocytometer (Kaya & Stock, 1997; Vélez et al. 1997).
Since only adults infected by M. anisopliae were detected, the experiment was performed with this fungus, extracted two months before inoculation and propagated in the medium SDLYM and with a control. A solution of 1x107 spores was applied. Twenty females and 20 males per treatment, same number for the control were evaluated; for a total of 80 experimental units. During 20 days a total of eight observations were made, each every second day; and during this observation time food and water was provided as necessary.
To test the pathogenic nematode effect, those recovered in the white grubs, belonging to the family Sterneinematidae (indentified using) (Kaya & Stock, 1997). Seventy two plastic vials, 36 for each treatment for each treatment, (nematode inoculation and control), with 6g of previously dried soil for 48 h received 3mL of distilled water. After 24 hours one E. longicollis larva and a corn grain was introduced in each vial. Larval instars were not determined at the start of the experiment and they varied from two-five instars. A nematode solution, containing 20Jl/cm3was prepared (Saenz, 2003), which has been the most effective treatment invading larvae of Clavipalpus ursinus (Coleoptera). To each vial 1cm3of the solution was applied; evaluations were performed every 72 hours for 15 days.
The soil was kept moist applying if necessary, every third day 1cm3of water. When noting dead larvae, the cadaver was extracted, washed with a solution of water and 0.5% hypochlorite, and placed in a modified White trap to confirm the presence of nematodes.
Data were analyzed with a qualitative approach and a descriptive- comprehensive scope, calculating and interpreting the larval and adult percent mortality.
RESULTS AND DISCUSSION
Symptoms with the treatments with Metharizium were dots and brown spots on the cuticle of the larva; the spots were observed 8-10 days before the insect's death. Later a slight change in the color of the larva was noted and a rigid consistency was detected. After death, a white mycelium initiated at the ventral side between the junction of the externits and pleurits. Confirmation of the fungal infection was made when an olive green sporulation was detected and under the microscope spores in columns appearance were observed.
When infected with Beauveria, the larva revealed a dark coloration at its anal area; dead individuals had a rigid consistency and the translucent larvae showed a whitish color. The initial appearance of mycelium showed where the dark spot was originally perceived, then covered the entire body. Beauveria infection was confirmed by the observation of the spores which provided a powdery appearance to the larva and by small spherical sacks covering the body (Posada & Pava-Ripoll, 2010). Under the microscope the globular spores, characteristics of this fungus, were detected.
Finally, in the case of pos. Paecilomyces the fungus did not produce spots, but a total color change of the whole dead body; the color ranged from intense yellow to salmon red. The larva was quite rigid and a few days after death, a rough texture of the cuticle was detected. The occurrence of white mycelium started between the abdominal segments; although no fungal sporulation was observed, the symptoms and signs were identical to those observed in the original specimens, from which the inoculum was taken.
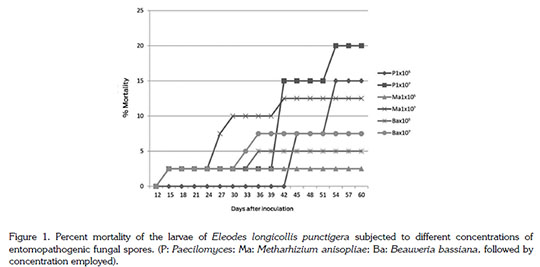
Figure 1 shows the percentage of larval mortality after 15 days of inoculation. Before this all larvae were alive, except for those exposed to 1x105of pos. Paecilomyces, all treatments produced the death of 5% of the 20 inoculated larvae. A maximum of 20% mortality throughout the trial was observed only with the high dose of this fungus at day 60. The concentration 1x105 achieved 15% mortality after two months. The higher dose, 1x107of M. anisopliae caused 12.5% mortality, while the same high dose of B. bassiana at day 60 showed only 7.5% mortality.

Only Paecilomyces revealed a tendency to increase mortality over time. The other pathogens did not improve their mortality rate, Metarhizium from day 42 and Beauveria from day 36 on after inoculation.
Although, in general terms, all pathogens initiated their action and killed E. longicollis larvae, it was required a relatively long time for an increase in larval mortality. It is known that the three fungi are not fast acting. The low efficiency may be due to a natural larval tolerance to the infection process, i.e. the penetration of spores through the cuticle. For this action, entomopathogenic fungi produce extracellular enzymes, whose function in the pathogenicity corresponds to the suppression of the immune system and dissolution of the insect cuticle, among others (Gomes Fernandes et al. 2012; Tiemi Ito et al. 2007). It is possible, that the production of these enzymes from the strains used in this work was not appropriate to achieve a proper penetration through the chitin constituting the cuticle of E. longicollis punctigerus.
The same consideration may be true for the low pathogenic action of M. anisopliae against males and females at 1x107. Mortality of the first insects was detected after six days, with three dead males and one dead female. Three days later increased to 12% mortality with one more insect of each sex, for a total of five males (25%) and three females (15%) dead.
In figure 2 results using the nematode, Steinernema sp., at a concentration of 20 infective juveniles / cm3is shown. At day three one dead individual was initially detected; at day ten 22.2% of dead larvae and at the end of the experiment 12 individuals representing 33.3%. The figure 2 shows a clear trend of increased larval mortality over time. Nansen et al. (2013) mention that the most important conditions for the development and the infection of another Tenebrionidae, Cynaeus angustus, by nematodes is soil moisture, which in this experiment was apparently adequate for the movement and action of the nematode. Maybe the relatively low efficiency has to do with the origin of the nematode, which was obtained from a beetle of another family, having a certain influence. Although nematodes are not considered specific, Geden et al. (1985) obtained satisfactory results controlling larvae of another tenebrionid, A. diaperinus, dispersing infective juveniles of Steinernema feltiae to the floor of poultry houses.
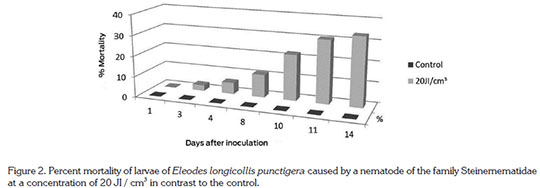
Comparing the maximum larval mortality produced by fungi and nematodes, it appears that the latter causes a faster and increased mortality of larvae of the false wire-worm, thus showing an advantage for the possible use within a management program of the insect.
The results of these preliminary experiments revealed a lower mortality rate as expected, based on previous field observations. Apparently, the insect is tolerant to infection by these fungi, at least under the conditions of the trial. In the case M. anisopliae, the low mortality rate can be attributed to adverse environmental conditions, since infection is influenced by temperature, moisture and particular conditions such as pH, organic matter content and texture, among other physical properties (Posada & Pava-Ripoll, 2010; Bidochka et al. 1998; Quesada-Moraga et al. 2007) not evaluated in this study. These and other experimental conditions also influence the efficiency of B. bassiana. Steinkraus et al. (1991) showed that the susceptibility to the fungus in the tenebrionid Alphitobius diaperinus, is influenced by the hosts instar, the substrate and the formulation of the inoculum.
In addition, it should be noted that the experiments were conducted with soil and although the presence of micro, meso and macro organisms were controlled, conditions such as the vials moisture, temperature and soil characteristics were difficult to control. They influence infection by microorganisms and the insects contact with the microorganism or its infective structures.
The contact of microorganisms with the host is a very important factor that should be taken into account when assessing its pathogenicity. In this context, low larval and adult susceptibility may be due to the low affinity of fungi with the rigid exoskeleton. In the case of nematodes, a certain soil humidity is required, sufficient to allow their movement through the soil and recognize the host to infect.
Another factor to consider, as mentioned above, is that the soil used was completely free of any organism, differing from natural conditions were mites, springtails and insects not susceptible to the infection of the microorganisms evaluated, could serve as means of transport so that larvae of the target species could come into contact with the pathogen employed.
From this preliminary and basic research, it can be concluded that both, the entomopathogenic fungi and the nematode could be part of an integrated management program of the false wire-worm, once a suitable dose of inoculation and the conditions of optimal field condition for adult and larval infection are established. Also it is considered important to study the molecular affinity of spores of entomopathogenic and the insects instar.
Acknowledgment: The authors thank the U.D.C.A for funding the research under the context of ''young researcher'', first author of this article. Conflicts of interest: This manuscript was prepared and revised with the participation of all authors, who declare that there is no conflict of interest that could put in danger the validity of the results.
BIBLIOGRAPHY
1. ALVES, S.B.; LOPES, R.B. 2008. Controle microbiano de pragas na América Latina; avances e desafíos. PIracicaba, FEALQ. 414p.
2. BIDOCHKA, M.J.; KASPERSKI, J.E.; WILD, G.A.M. 1998. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitat. Can. J. Botany. 76(7):1198-1204.
3. BLAISDELL, F.E. 1935. New Species of Eleodes from Mexico in the British Museum (Col: Tenebrionidae). Stylops: J. Taxonomic Entomol. 4(7):156-160.
4. CALKINS, C.O.; KIRK, V.M. 1973. Food preference of a false wireworm, Eleodes suturalis. Environ. Entomol. 2:105-108.
5. ERICSSON, J.D.; KABALUK, J.D.; GOETTEL, M.S.; MYERS, J.H. 2007. Spinosad interacts synergistically with the insect pathogen Metarhizium anisopliae against the exotic wireworms Agriotes lineatus and Agriotes obscurus (Coleoptera: Elateridae). J. Econ. Entomol. 100(1):31-38.
6. GEDEN, C.J.; ARENDS, J.J.; AXTELL, R.C. 1987. Field trials of Steinernemia feltiae (Nematoda: Steinernematidae) for control of Alphitobius diaperinus (Coleoptera: Tenebrionidae) in commercial broiler and turkey houses. J. Econ. Entomol. 80:136-141.
7. GEDEN, C.J.; AXTELL, R.C.; BROOKS, W.M. 1985. Susceptibility of the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae) to the entomogenous nematodes Steinernema feltiae, S. glaseri and Heterorhabditis heliothidis (Heterorhabditidae). J. Entomol. Sci. 20:331-339.
8. GOMES FERNANDES, E.; MAIA VELÉRIO, H.; FELTRIN, T.; VAN DER SAND, S.T. 2012. Variability in the production of extracellular enzymes by entomopathogenic fungi grown on different substrates. Braz. J. Microbiol. 43:827-833.
9. HANSON, P. 1993. Control Biológico de Insectos. Edit. CATIE. Turrialba (Costa Rica). 40p.
10. KABALUK, J.T.; ERICSSON, J.D. 2007. Environmental and behavioral Constraints on the infection of wireworms by Metarhizium anisopliae Environ. Entomol. 36(6):1415-1420.
11. KAYA, H.K.; STOCK, S.P. 1997. Techniques in insect nematology. In: Lacey, L.A. (ed.) Manual of techniques in insect pathology. Biological Techniques Series. San Diego, London: Academic Press, p.281-324.
12. LACEY, L.A.; KAYA, H.K. 2007. Field Manual of Techinques in Invertebrate Pathology. Edit. Springer. Países Bajos. 868p.
13. MAGGIORANI, A.; GUDIÑO, S. 1996. Uso de nematodos entomopatógenos como una alternativa para el control de insectos plagas. EN: FONAIAP Divulga. No 53. Abril-Junio.
14. NANSEN, C.; STOKES, B.; JAMES, J.; PORTER, P.; SHIELDS, E.J.; WHEELER, T.; MEIKLE, W.G. 2013. Biological control agent of larger black flour beetles (Coleoptera: Tenebrionidae): a nuisance pest developing in cotton gin trash piles. J. Econ. Entomol. 106(2):648-652.
15. NICHOLLS ESTRADA, C.I. 2008. Control Biológico de Insectos con un Enfoque Agroecológico. Edit. Universidad de Antioquia. Medellín (Colombia) 282p.
16. PEARS, F.B. 2009. High Plants Integrated Pest Management: False Wireworm. Disponible desde internet en: http://wiki.bugwood.org/HPIPM:False_Wireworm (con acceso 02/09/12).
17. PEDRINI, N.; VILLAVERDE, M.I.; FUSE, C.B.; DAL BELLO, G.M.; JUÁREZ, M.P. 2010. Beauveria bassiana infection after colony development and defensive secretions of the beetles Tribolium castaneum and Ulomoides dermestoides (Coleoptera: Tenebrionidae). J. Econ. Entomol. 103(4):1094-1099.
18. POSADA F., F.J.; PAVA-RIPOLL, M. 2010. Enemigos Naturales de la Broca del café con énfasis en los Hongos Entomopatógenos. EdiU.D.C.A. Bogotá. Colombia. 239p.
19. QUESADA-MORAGA, E.; NAVAS-CORTÉS, J.A.; MARANHAO, E.A.A.; ORTIZ-URQUIZA, A.; SANTIAGOALVAREZ, C. 2007. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 111:947-966.
20. QUIROGA-MURCIA, D.; POSADA-FLÓREZ, F.J. 2013. Daño ocasionado por el falso gusano alambre Eleodes pos. omissoides Blaisdell (Coleoptera: Tenebrionidae) en semillas de gramíneas y leguminosas. Rev. U.D.C.A Act. & Div. Cient. 16(2):391-400.
21. ROGERS, L.E.; WOODLEY, N.E.; SHELDON, J.K.; BEEDLOW, P.A. 1988. Diets of darkling beetles (Coleoptera: Tenebrionidae) within a Shrub-Steppe ecosystem. Ann. Entomol. Soc. Am. 81:782-791.
22. ROSALES, C.L.; RODRÍGUEZ, H.M.G.; ENRIQUE, R.; PUENTES, L.; GARCÍA, J. 2009. Cría Masiva de Nematodos Entomopatógenos para el Control de Insectos Plagas. En: INIA Divulga 12. p.19-22.
23. SAENZ, A. 2003. Eficacia de invasión de Tecia solanivora y Clavipalpus ursinus por el nematodo Steinernema feltiae. Manejo Integrado de Plagas y Agroecología. 67:35-43.
24. SAUNDERS, L.J.; COTO, T.D.; KING, B.S.A. 1998. Plagas Invertebradas de Cultivos Anuales Alimenticios en América Central. Edit. CATIE. Turrialba (Costa Rica). 305p.
25. STEINKRAUS, D.C.; GEDEN, C.J.; RUTZ, D.A. 1991. Suscpetibility of lesser mealworm (Coleoptera: Tenebrionidae) to Beauveria bassiana (Moniliales: Moniliaceae): effects of host stage, substrate, formulation, and host passage. J. Med. Entomol. 28(3):314-321.
26. TIEMI ITO, E.; VARÉA-PEREIRA, G.; TOMOE MIYAGUI, D.; PIMENTA PINOTTI, M.H.; OLIVEIRA JANEIRO NEVES, P.M. 2007. Production of extracellular proteases by Brazilian strains of Beauveria bassiana reactivated on coffee Berry borer, Hypothenemus hampei. Braz. Arch. Biol. Technol. 50(2):217-223.
27. TRIPLEHORN, C.A. 2010. Notes on three species of Eleodes Eschenholtz (Coleoptera: Tenebrionidae) and description of a new species. Coleopts. Bull. 64:373-378.
28. TRIPLEHORN, C.A.; CIFUENTES RUIZ, P. 2011. A new species of Eleodes (Eleodes) from Mexico, with ecological y phonological notes (Coleoptera: Tenebrionidae). Zootaxa. 2937:66-68.
29. TRIPLEHORN, C.A.; THOMAS, D.B. 2011. Studies in the genus Eleodes Eschscholtz with a revision of the subgenus Melaneleodes Blaisdell and Omegeleodes, new subgenus (Coleoptera: Tenebrionidae: Eleodini). Transact. Am. Entomol. Soc. 137:251-281.
30. VÉLEZ A., P.E.; POSADA F., F.J.; MARÍN, P.; BUSTILLO P., A.E.; GONZALES G., M.T.; OSORIO, E. 1997. Técnicas para el control de calidad de formulaciones de hongos entomopatógenos. Boletín Técnico, N°17. Cenicafé (Colombia). 37p.
31. VIEIRA TIAGO, P.; TINTI DE OLIVEIRA, N.; ALVES LIMA, E.A. d. L. 2014. Biological insect control using Metarhizium anisopliae: morphological, molecular and ecological aspects. Ciência Rural. 44(4):645-651.
32. ZENNER DE POLANÍA, I.; QUIROGA-MURCIA, D.E.; GÓMEZ PIEDRAS, J.J.; BLANCO, C.A. 2014. A false wireworm (Coleoptera: Tenebrionidae) acting as a cutworm of tomato plants in greenhouses. Southwestern Entomologist. 39(1):37-47.
Received: 22 October 2015 Accepted: 28 March 2016
Revista U.D.C.A Actualidad & Divulgación Científica por Universidad de Ciencias Aplicadas y Ambientales se distribuye bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.