CIENCIAS AGROPECUARIAS-Artículo Científico
SYNTHESIS, CHARACTERIZATION AND ANTIMICROBIAL ACTIVITY OF A Pd(II) COMPLEX WITH A 1,3-DIPHENYLPYRAZOLE-4-CARBOXALDEHYDE THIOSEMICARBAZONE LIGAND
SÍNTESIS, CARACTERIZACIÓN Y ACTIVIDAD ANTIMICROBIAL DE UN COMPLEJO DE Pd(II) CON LIGANTE 1,3-DIFENILPIRAZOL-4-CARBOXALDEHÍDO TIOSEMICARBAZONA
Ana E. Burgos C.1, Lenka Tamayo2, Rosabel Torrellas-Hidalgo3
1Ph.D. Ciencias-Química, M.Sc. Química, Profesora Asociada en Dedicación Exclusiva, Grupo de Investigación en Química de Coordinación y Bioinorgánica, Departamento de Química, Facultad de Ciencias, Universidad Nacional de Colombia, Av. Cra 30 No. 45-03, Bogotá D.C., Colombia. E-mail: aeburgosc@unal.edu.co
2M.Sc. Química, Química, Grupo de Investigación en Química de Coordinación y Bioinorgánica, Departamento de Química, Facultad de Ciencias, Universidad Nacional de Colombia, Av. Cra 30 No. 45-03, Bogotá D.C., Colombia. E-mail: levitalo616@gmail.com
3Ph.D. Química, Química, Udana Inc.14027 Memorial Drive # 349, Houston, TX, 77079.USA. E-Mail: rosabel.torrellas@gmail
Rev. U.D.C.A Act. & Div. Cient. 17(2): 477-486, Julio-Diciembre, 2014
SUMMARY
This article describes the synthesis and characterization of a new complex, [Pd(Ph2PzTSC)2], formed between palladium(II) and 1,3-diphenylpyrazole-4-carboxaldehyde thiosemicarbazone ligand as a strategy for antimicrobial activity improvement the synthesized complex. The metal coordination leads to an improvement of ligand pharmacological activities and synergistic effects involving both metal ion as the ligand. The bidentate ligand is coordinated to metal ion through the azomethine nitrogen atoms and the sulphur in the form of thiol by deprotonation of the NH-C=S group. The antimicrobial activity of these new compounds was evaluated against gram-negative (Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa) and gram-positive (Staphylococcus aureus and Bacillus thuringiensis) bacteria and two yeasts strains (Candida albicans and Saccharomyces cerevisiae). A comparison between the antimicrobial activity of the complex and that of the free ligand revealed that the coordination of Pd(II) improved the activity.
Key words: Schiff base ligands, Pd(II) compounds, biological activity.
RESUMEN
En este artículo se describe la síntesis y caracterización de un nuevo complejo de paladio(II), [Pd(Ph2PzTSC)2], con el ligante 1,3-difenilpirazol-4-carboxaldehído tiosemicarbazona (Ph2PzTSC) como una estrategia para mejorar la actividad antimicrobiana del complejo formado. La coordinación del ion metálico y el efecto sinérgico entre estos, conduce a mejorar la actividad biológica del complejo. El ligante se coordina al ion metálico de modo bidentado a través del átomo de nitrógeno del azometino y del azufre en forma de tiol, por la desprotonación del grupo NH-C=S. La actividad antimicrobiana de estos compuestos fue evaluada frente a bacterias Gram-negativas (Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa) y Gram-positivas (Staphylococcus aureus, Bacillus thuringensis) y dos levaduras (Candida albicans y Saccharomyces cerevisiae). Cuando fueron comparados los resultados de la actividad antimicrobiana con la actividad del ligante libre, se observó que la coordinación del Pd(II) mejoró la actividad.
Palabras clave: Ligantes bases de Schiff, compuestos de Pd(II), actividad biológica.
INTRODUCTION
Thiosemicarbazones are an important and versatile type of ligands due to the potential donor atoms that they possess, among which sulfur is of paramount importance in the metal-ligand linkage (Matesanz et al. 2013). These are strong metal chelating agents and moreover some of them have showed antineoplastic activity by themselves. It has been demonstrated that the biochemical mechanism of action involves, among others, ribonucleotide reductase inhibition and non-covalent DNA binding (Lobana et al. 2009; Matesanz & Souza, 2009). Thiosemicarbazones also have wide pharmacological versatility and cytotoxic, antitumor, antimalarial, antimicrobial, and antiviral properties (Da Silva et al. 2013a). The thiosemicarbazones and their complexes with transition metals are of great chemical and biological interest because they exhibit diverse pharmacological actions, which include antibacterial, antifungal, antitumor, antiviral, and antimalarial activities, among others (Husain et al. 2007; Beraldo & Gambino, 2004; Salman & Mohamad, 2009; Matesanz et al. 2013; Rebolledo et al. 2005; Da Silva et al. 2013a; Pelosi, 2010). On the other hand, the pyrazole nucleus and its derivatives exhibit a diverse range of pharmacological activities, presenting cytotoxic, antitumor, analgesic, antiinflammatory, antipyretic, antibacterial, antioxidant, antiviral, anticonvulsant, muscle relaxant and antifungal properties, and hypoglycemic effects (Leovac et al. 2007; Hashimoto et al. 2002; Habeeb et al. 2001; Price & Jorgensen, 2000; Ali et al. 2012). Due to its wide range of biological activity, pyrazole ring constitutes a relevant functional group in pharmaceutical industry. In fact, such a heterocyclic moiety represents the core structure for number of drugs (Anoop & Ranab, 2010). Moreover, the various biological activities exhibited by Pd(II) complexes with ligands of electron donor atoms, such as nitrogen and sulfur in the thiosemicarbazones, have prompted the study of these compounds (Gambino et al. 2007; Garoufis et al. 2009).
This work is aimed to describe the synthesis and chemical characterization of a new Pd(II) complex obtained by the reaction of Ph2PzTSC with PdCl2 as a strategy for antimicrobial activity improvement of the synthesized complex. The antimicrobial activity of both the new compound synthesized and the corresponding ligand against gram-negative and grampositive bacteria and two yeasts strains has been evaluated. In many cases, metal coordination leads to an improvement of thiosemicarbazone pharmacological activities and synergistic effects involving both metal and the thiosemicarbazone have been reported (Da Silva et al. 2013b).
MATERIALS AND METHODS
These compounds were characterized using spectroscopic techniques (infrared, FTIR and mono and two dimensional nuclear magnetic resonance, NMR). Fast atom bombardment (FAB) mass spectrometry was also used to elucidate the structure of the Pd(II) complex. The antimicrobial activity of the synthesized compounds was evaluated against grampositive and gram-negative bacteria and two yeasts strains.
Melting points of the synthesized compounds were determined using Mettler FP90 equipment with central processing, heating cell FP82HT and microscope Olympus CH-2 with digital calibration. IR spectra were obtained on a Shimadzu model 8400/8900 FTIR instrument over the wave- number range of 4000 to 250 cm-1 and on a Nicolet iS10 Spectrometer (Thermo Fisher Scientific). The samples for FTIR were prepared in KBr pellets. The 1H NMR, 13C NMR, and bidimensional spectra were obtained with a Bruker Ultra- shieldTM 400 MHz NMR, using TMS as internal standard and DMSO-d6 as a deuterated solvent. The high resolution mass spectrum with an EBE configuration was captured on an AutoSpec M is a forward geometry; configuration was obtained using a UTOSPEC-Q device. It was used for positive ion FAB analysis, and thioglycerol was used as the ionization matrix. Antimicrobial assays were performed with the following microorganisms: i) gram-positive bacteria: Staphylococcus aureus ATCC 29213 and Bacillus thuringiensis sp.; ii) gramnegative bacteria: Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, and Pseudomonas aeruginosa sp.; and iii) yeasts: Candida albicans ATCC 10231 and Saccharomyces cerevisiae ATCC 38626.
Synthesis of Ph2PzTSC ligand: The Ph2PzTSC ligand was prepared according to the procedure of Leovac et al. (2007) using a molar ratio of 1:1. The percentage yield was 80%, which is similar to the yield reported in the literature (Leovac et al. 2007).
The Ph2PzTSC compound exhibited the following properties: M.P.: 216°C (reported 217°C). Yield: 80%. IR (KBr)/cm-1: (NH2 asym and sym), 3143 (NH hydrazine), 1590 (C=N azomethine), 1551 (C=N pyrazole), 1209 (NHC=S), 1096 (C=S). 1H NMR (400 MHz, DMSO-d6): d 7.39 (t, 1H, J 7.2 Hz, Hp '), 7.45-7.60 (m, 5H, Hm', Hm, Hp), 7.69 (d, 2H, Ho), 7.76 (brs, 1H, H-NH2), 7.91 (d, 2H, J 7.7 Hz, Ho’), 8.22 (brs, 1H, H-NH2 ), 8.24 (s, 1H, CH=N), 9.18 (s, 1H, H-5 pz), 11.33 ppm (s, 1H, NH-C=S). 13C NMR (400 MHz, DMSO-d6): δ 117.7 (C-4 pz), 118.9 (Co'), 127.4 (Cp'), 128.1 (C-5pz), 128.6 (Co), 129.0 (Cp), 129.2 (Cm), 130.1 (Cm'), 132.5 (Ci), 135.4 (CH=N), 139.5 (Ci’), 151.8 (C-3pz), 178.0 (C=S).
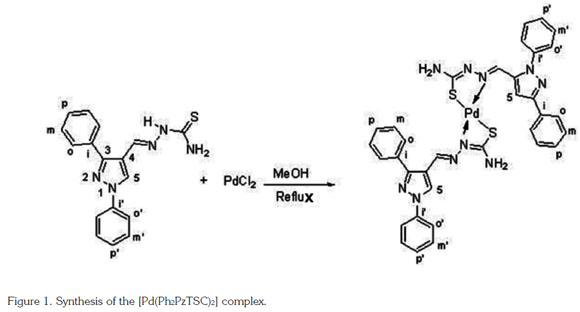
Synthesis of [Pd(Ph2PzTSC)2] Complex: The [Pd(Ph2PzTSC)2] complex (Figure 1), was synthesized using a 1:1 metal-ligand molar ratio. Palladium(II) chloride (PdCl2, 27.6 mg, 0.15 mmol) was dissolved by heating in 10 mL of methanol and was subsequently added to a hot solution of Ph2PzTSC (50 mg, 0.15 mmol) in 10mL of acetone. The mix ture was kept under continued reflux for 24 h. An orange solid was obtained, and it was washed several times with methanol to remove the excess metal. The solid was subsequently dried with anhydrous calcium chloride, and the percent yield was 49%. The complex is stable at room temperature (19 ± 1 °C) and is soluble in DMSO. The [Pd(Ph2PzTSC)2] complex decomposed at 290 °C. Yield: 49%. IR (KBr)/cm-1: 3250-3423 (NH2 asym and sym), 1615 (C=N azomethine), 1594 (C=N pyrazole), 1051 (NN), 756 (CS), 550 (Pd-N). 1H NMR (400 MHz, DMSO-d6): δ 7.41 (t, 4H Hp' and H-NH2), 7.48-7.58 (m, 12H, H-NH2, Hm', Hm, Hp), 7.65 (d, 4H, J 7.7 Hz, Ho), 7.97 (d, 4H, J 7.7 Hz, Ho'), 8.36 (s, 1H, CH=N), 9.46 (s, 1H, H-5 pz). 13C NMR (400 MHz, DMSO-d6): δ 112.8 (C-4 pz), 119.1 (Co'), 127.3 (Cp'), 128.6 (Cm), 128.8 (Cp, Co), 129.5 (Cm'), 131.1 (Ci), 133.0 (C-5 pz), 138.7 (Ci'), 144.2 (CH=N), 154.1 (C-3 pz), 172.2 (C-S).

Antimicrobial activity assays: The antibacterial activity assays were performed using the disk diffusion method in a Petri dish. The culture medium used was TSA (Trypticase Soy Agar) for bacteria and PDA (Potato Dextrose Agar, Scharlau) for yeasts. The minimum inhibitory concentration (MIC) method was used to estimate the lowest inhibitory concentration (in µg/mL or ppm) based on the methodology of Tamayo et al. (2014).
Twenty microliters of each test compound (Ph2PzTSC, PdCl2 and [Pd(Ph2PzTSC)2]) dissolved in DMSO in a concentration range between 50 and 500 ppm (282 - 2820 µM) for PdCl2, 155 - 1556 µM for Ph2PzTSC, 66 - 669µM for [Pd(Ph2PzTSC)2], were dissolved in DMSO and placed in 7mm-diameter wells, were the microorganisms under evaluation were grown (~108 UFC/mL) in their respective culture media. The microbial culture was incubated at 35°C for 24 h for the bacterial strains and at 20°C for 72 h for yeast strains. DMSO was used as a control solvent. Cephalothin (40 mg/mL) was utilized as a positive control for all bacterial strains, except for P. aeruginosa, where ciprofloxacin (0.3%) was used. Clotrimazole (1%) was used for the cultivation of yeasts. The susceptibility of microorganisms to each of the tested compounds was determined through observation of the formation of an inhibition zone around each well on agar, which was measured in millimeters.
RESULTS AND DISCUSSION
The IR spectrum of the Ph2PzTSC ligand showed the characteristic bands of the compound reported in the literature (Leovac et al. 2007). These observations confirmed the formation of the thiosemicarbazone compounds (Figure 1).
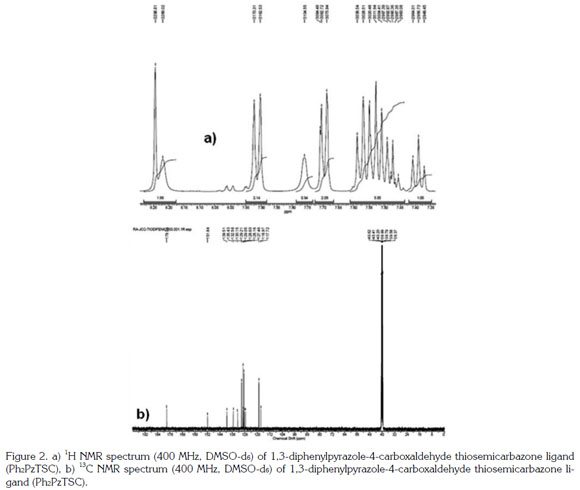
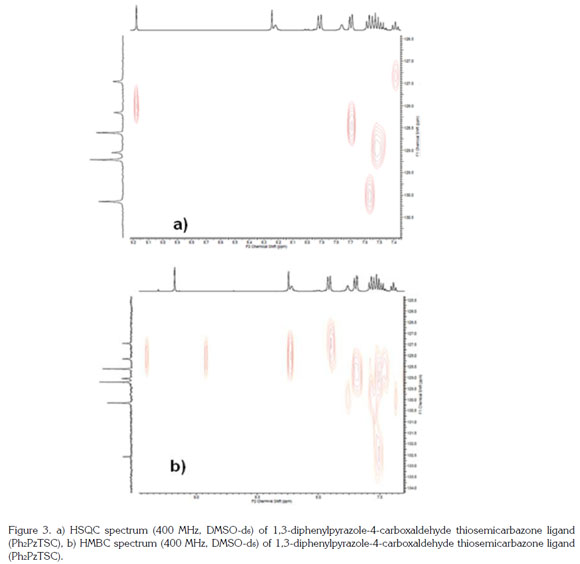
The 1H NMR spectrum of the Ph2PzTSC ligand in DMSO-d6 at 200 MHz has been reported by Leovac et al. (2007). The spectrum of the compound synthesized here was obtained using a 400 MHz instrument with DMSO-d6 and produced the following signals: triplet at 7.39ppm integrated to 1 proton (Hp) with J 7.2 Hz; multiplet at 7.45-7.60 ppm integrated to 5 protons (Hm, Hm, Hp); doublet at 7.69ppm with J 7.6 Hz integrated to 2 protons (Ho); broad singlet at 7.76 ppm corresponding to 1 of the 2 non-equivalent protons of the NH2 group; double at 7.91ppm with J 7.7 Hz, integrated to 2 protons (Ho); broad singlet at 8.22ppm corresponding to the other not-equivalent proton of the NH2 group; singlet at 8.24ppm integrated to the proton of the azomethine group (CH = N); singlet at 9.18ppm integrated to 1 proton corresponding to H-5 Pz, and a downfield singlet at 11.33ppm integrated to the proton of the NH-C=S group (Figure 2a). The 13C NMR spectrum showed the 13 carbon signals expected for the reported structure (Figure 2b), with chemical shifts similar to those described in the literature (Leovac et al. 2007). Dimensional experiments (HSQC and HMBC) confirmed the proposed structure (Figure 3).
[Pd(Ph2PzTSC)2] complex: The IR spectrum of the [Pd(Ph2PzTSC)2] complex did not show the stretching band of the n(NH) group at 3150 cm-1 attributed to hydrazine, which suggests the possible deprotonation of this group (Leovac et al. 2007). Additionally, the presence of a new band of lower intensity was observed at 1051cm-1, which was attributed to the stretching band of a n(NN) group; the presence of this band confirmed the deprotonation of the n(NH) group of hydrazine. The IR spectrum of the Pd(II) complex showed a lower intensity band at 550cm-1 corresponding to n(Pd-N) (Gambino et al. 2007). Furthermore, no bands were observed in the region between 305 and 335cm-1, which is normally associated with n(Pd-Cl) stretching; the lack of these bands suggests that the ligand was not in the coordination sphere. Additionally, the bands of the azomethine (C=N) and thiocarbonyl (C-S) groups were shifted, which suggests that the coordination of the ligand to the metal ion occurred through the nitrogen and the sulfur atoms, respectively.
When comparing the chemical shifts in the 1H NMR spectrum of the free ligand (Ph2PzTSC) with the complex ([Pd(Ph2PzTSC)2]) in [DMSO-d6] (Figure 4), no chemical shift for the proton of the NH-C=S group was observed. This result suggested that the ligand was thiolated and eliminates the possibility of a structure in which the ligand is in the tautomeric thione. The upfield chemical shifts of the amino group protons overlapped the multiplet in the region between 7.48 and 7.58ppm and the triplet at 7.41ppm, which suggests that the electron density on the thioamide group (-CSNH2) changed due to the thione-thiol modification of the thiocarbonyl group. In addition, a 0.1ppm (Δδ) downshift shift of the proton signal of the azomethine group relative to the position of the signal of the free ligand was observed, which suggests a lower electron density on the azomethine group and indicates that the metal is coordinated through the available electron pair of the nitrogen atom of that group.
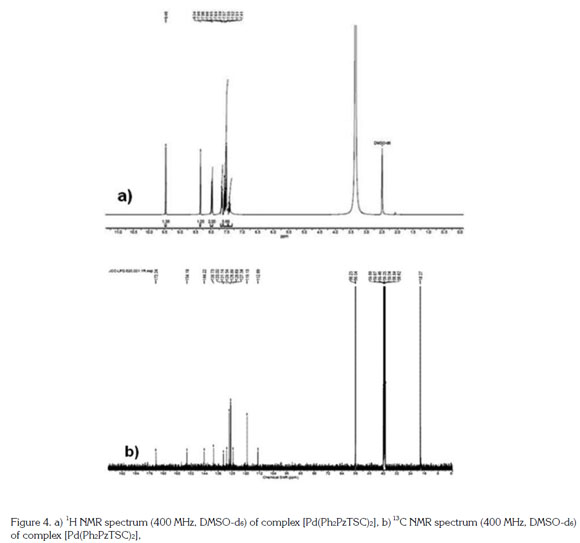
Assignments of the signals in the 13C NMR spectrum are based on the chemical shifts and intensity patterns and coordination induced shift (CIS) of the carbon signals in the complex, in comparison to those of the free ligand, Δδ = δ (complex) - δ (free ligand).
The 13C NMR spectrum of the [Pd(Ph2PzTSC)2] complex (Figure 4) showed a signal at 172.6ppm. A comparison of this signal with the chemical shift of the free ligand (178.0ppm) revealed an upfield shift of 5.4ppm (Δδ). This spectroscopic evidence indicates that the Pd(II) is coordinated to the sulfur atom of the thiocarbonyl group in its thiol form (Leovac et al. 2007; Casas et al. 1998; Rodríguez-Argüelles et al. 1995).
Moreover, a downfield shift of the carbon of the azomethine group at 144.2ppm and Δδ= 8.8ppm compared with the signal of carbon in the free ligand occurred. This spectroscopic evidence indicates a lower electron density on the azomethine group and suggests that the metal is coordinated to the electron pair of the nitrogen in the azomethine group (Leovac et al. 2007).
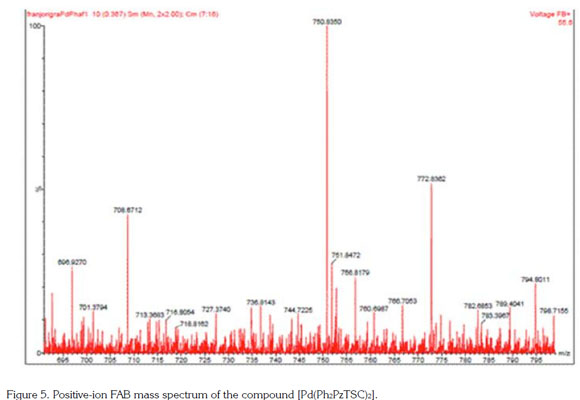
The positive ion FAB mass spectrum of the [Pd(Ph2PzTSC)2] complex (Figure 5), showed a m/z peak at 750.8350 related to the fragment [M + 3H]+, a new peak at m/z 772.8362 related to the fragment [M + Na + 3H], and another peak at m/z 789.4041 related to the fragment [M + K + 3H]. These species originated from fragmentation, involve binding or loss of some hydrogen atoms in these fragments. This complex, [Pd(Ph2PzTSC)2], was prepared in a 1:1 metal-ligand molar ratio, however the complex obtained presented a 2:1 metal-ligand molar ratio. This is indicated by the FAB mass spectrum, as the peak of major abundance was observed at an m/z 750.8350, confirming the molecular mass of the compound. Taking into account this mass-charge ratio and that obtained by 1H and 13C NMR spectra the purity, these results confirm that the [Pd (Ph2PzTSC)2] complex was successfully formed.
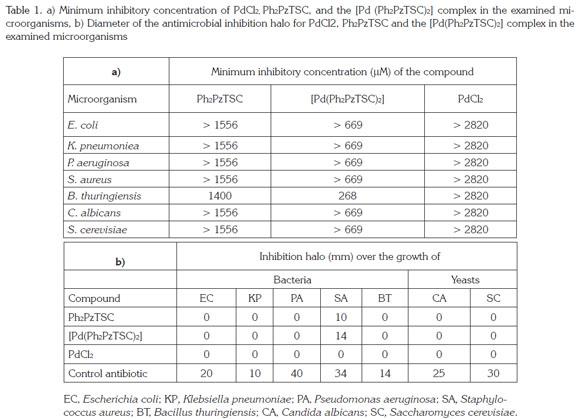
Antimicrobial activity assays: The compounds Ph2PzTSC and [Pd(Ph2PzTSC)2] did not demonstrate antifungal activity in the range of concentrations used. Furthermore, the metal precursor (PdCl2) did not exhibit neither antimicrobial nor antifungal activity in the concentration range used (Table 1).
The Ph2PzTSC ligand exhibited antibacterial activity inhibition only for Staphylococcus aureus, with a minimum inhibitory concentration of 1400 µM and with an inhibition halo of 10mm (Table 1). The complex [Pd(Ph2PzTSC)2] showed activity for the gram-positive bacteria S. aureus (Table 1a, b). A minimum inhibitory concentration (MIC) of 268µM was observed for S. aureus with an inhibition halo of 14mm. The results suggest that the antimicrobial activity occurs due to synergy between the metal and the ligand. This finding indicated that the metal ion improved the antibacterial activity compared to the inhibition of the free ligand at a concentration of 1400µM. This behavior can be explained by the Overtone's concept of cell permeability (Prabhakaran et al. 2008) and Tweedy's chelation theory (Tweedy, 1964). According to the Overtone's concept, the lipid membrane that surrounds the cell facilitates the passage of only lipid-soluble materials; therefore, the lipid is an important factor that controls the antimicrobial activity. Tweedy's chelation theory, explains that the ion polarity of Pd(II) is reduced by overlapping of the ligand orbitals and the exchange of the partial positive charge of the metal ion to the donor atoms of the ligand; thereby, the delocalization of π-electrons increases on the chelate ring and improves the lipophilicity of the complexes.
The increment in lipophilicity that increases the penetration of the complex into the lipid's membrane and blocks the metal binding sites in the microorganisms' enzymes might disturb the process of cell respiration and the synthesis of proteins, thereby inhibiting the growth of the organism (Prabhakaran et al. 2008).
The results of the antimicrobial activity (Table 1a, b) indicated that the synthesized compounds exhibit a smaller halo of inhibition with respect to the positive controls used, which suggests that the compounds are less bioavailable. However, considering the concentrations used for the antimicrobial controls in the biological testing, it can be seen that the synthesized Pd(II) complex has a high growth inhibitory effect on the gram-positive bacteria S. aureus. For example, for S. aureus a concentration of 268µM of the [Pd(Ph2PzTSC)2] complex originated a 14mm zone of inhibition, while for the same bacteria the positive antimicrobial control used (cephalothin) showed a 34mm zone of inhibition but a 373 times higher concentration (100'000µM). For free ligand, at the concentration used (1400µM), and this inhibition was also higher (>70 times) that the positive antimicrobial control used.
These antimicrobial tests show an interesting of newly synthesized [Pd(Ph2PzTSC)2] complex to be used as an antimicrobial compound specifically to gram-positive bacteria (S. aureus). This complex should also used in biological assays to observe its effect against tumor cells, since similar Pd(II) compounds have shown promising effects against these type of cells (Husain et al, 2007; Beraldo & Gambino, 2004; Kavala-Demertzi et al. 2013; Kovala-Demertzi et al. 1999; Quiroga et al. 1998; Tamayo et al. 2014).
A novel Pd(II) complex with the 1,3-diphenylpyrazole-4-carboxaldehyde thiosemicarbazone (Ph2PzTSC) ligand was synthesized and characterized. According to the results obtained using different techniques, the complex exhibits ambidentate coordination through nitrogen and sulfur atoms in the thiol form. The results of various spectroscopic techniques and mass spectrometry (FAB) confirm the proposed structure for the compound.
The synthesized compounds showed antimicrobial activity only against gram-positive bacteria S. aureus and this activity was higher than that of the antibiotic controls. The formation of the metal complex induces significant changes in the antimicrobial activity of the free ligand.
Acknowledgments: The authors wish to acknowledge financial support from the Dirección de Investigación, Sede Bogotá (DIB, Bogotá Research Directorate), Project No.201010016735, and the Vicerrectoría de Investigación for financial support for the translation of this manuscript through the ''Convocatoria Nacional Fortalecimiento de la Visibilidad de Producción Académica mediante el apoyo para traducción de estilo de artículos de investigación 2011-2012'', both from the National University of Colombia. We also thank Professor Milton Crosby, Faculty of Science in the Department of Pharmacy at the National University of Colombia, by supplying the strains used in the bioassays. Conflict of interest: This paper was prepared with the participation of all the authors, who declare that no conflict of interest that threatens the validity of the results presented.
REFERENCES
1. ALI, I.; WANI, W.A.; KHAN, A.; HAQUE, A.; AHMAD, A.; SALEEM, K.; MANZOOR, N. 2012. Synthesis and synergistic antifungal activities of a pyrazoline based ligand and its copper(II) and nickel(II) complexes with conventional antifungals. Microb. Pathog. 53:66-73.
2. ANOOP, S.; RANAB, A.C. 2010. Synthesis and anticonvulsant activity of 1-[(4, 5-dihydro-5-phenyl-3-(phenylamino)pyrazol-1-yl)]ethanone derivatives. J. Chem. Pharm. Res. 2(1): 505-511.
3. BERALDO, H.; GAMBINO, D. 2004. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini. Rev. Med. Chem. 4(9):31-39.
4. CASAS, J.S.; RODRIGUEZ-ARGÜELLES, M.C.; RUSSO, U.; SANCHEZ, A.; SORDO, J.; VAZQUEZ-LOPEZ, A.; PINELLI, S.; LUNGHI, P.; BONATI, A.; ALBERTINI, R. 1998. Diorganotin(IV) complexes of pyridoxal thiosemicarbazone: synthesis, spectroscopic properties and biological activity. J. Inorg. Biochem. 69(4):283-292.
5. DA SILVA, J.G.; DESPAIGNE, A.A.R.; LOURO, S.R.W.; BANDEIRA, C.C.; SOUZA-FAGUNDES, E.M.; BERALDO, H. 2013a. Cytotoxic activity, albumin and DNA binding of new copper(II) complexes with chalcone-derived thiosemicarbazones. Europ. J. Med. Chem.65:415-426.
6. DA SILVA, J.G.; PERDIGÃO, C.H.; SPEZIALI, N.; BERALDO, H. 2013b. Chalcone-derived thiosemicarbazones and their zinc(II) and gallium(III) complexes: spectral studies and antimicrobial activity. 66(3):385-401.
7. GAMBINO, D.; OTERO, L.; VIEITES, M.; BOIANI, M.; GONZÁLEZ, M.; BARAN, E.J.; CERECETTO, H. 2007. Vibrational spectra of palladium 5-nitrofuryl thiosemicarbazone complexes: Experimental and theoretical study. Spectrochim. Acta Part A. 68(2):341-348.
8. GAROUFIS, A.; HADJIKAKOU, S.K.; HADJILIADIS, N. 2009. Palladium coordination compounds as antiviral, anti-fungal, anti-microbial and anti-tumor agents. Coord. Chem. Rev. 253(9-10):1384-1397.
9. HABEEB, A.G.; RAO, P.N.P.; KNAUS, E.E. 2001. Design and synthesis of celecoxib and rofecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: replacement of sulfonamide and methylsulfonyl pharmacophores by an azido bioisostere. J. Med. Chem. 44(18):3039-3042.
10. HASHIMOTO, H.; IMAMURA, K.; HARUTA, J.I.; WAKITANI, K. 2002. 4-(4-cycloalkyl/aryl-oxazol-5-yl) benzenesulfonamides as selective cyclooxygenase-2 inhibitors: enhancement of the selectivity by introduction of a fluorine atom and identification of a potent, highly selective, and orally active COX-2 inhibitor JTE-522. J. Med. Chem. 45(7):1511-1517.
11. HUSAIN, K.; ABID, M.; AZAM, A. 2007. Synthesis, characterization and antiamoebic activity of new indole- 3-carboxaldehyde thiosemicarbazones and their Pd(II) complexes. Eur. J. Med. Chem. 42(10):1300-1308.
12. KOVALA-DEMERTZI, D.; GALANI, A.; MILLER, J.R.; FRAMPTON, C.S.; DEMERTZIS, M. 2013. Synthesis, structure, spectroscopic studies and cytotoxic effect of novel palladium(II) complexes with 2-formylpyridine-4-Nethyl-thiosemicarbazone: Potential antitumour agents. Polyhedron. 52:1096-1102.
13. KOVALA-DEMERTZI, D.; MILLER, J.R.; KOURKOUMELIS, N.; HADJIKAKOUA, S.K.; DEMERTZIS, M.A. 1999. Palladium(II) and platinum(II) complexes of pyridine-2-carbaldehyde thiosemicarbazone with potential biological activity. Synthesis, structure and spectral properties. Extended network via hydrogen bond linkages of [Pd(PyTsc)Cl]. Polyhedron. 18(7):1005-1013.
14. LEOVAC, V.M.; NOVAKOVIC, S.B.; BOGDANOVIC, G.A.; JOKSOVIC, M.D.; SZÉCSÉNYI, K.M.; CESLJEVIC, V.I. 2007. Transition metal complexes with thiosemicarbazide-based ligands. Part LVI: Nickel(II) complex with 1,3-diphenylpyrazole-4-carboxaldehyde thiosemicarbazone and unusually deformed coordination geometry. Polyhedron. 26: 3783-3792
15. LOBANA, T.S.; SHARMA, R.; BAWA, G.; KHANNA, S. 2009. Bonding and structure trends of thiosemicarbazone derivatives of metals-An overview. Coord. Chem.. Rev. 253(7-8):977-1055.
16. MATESANZ, A.I.; LEITAO, I.; SOUZA, P. 2013. Palladium(II) and platinum(II) bis(thiosemicarbazone) complexes of the 2,6-diacetylpyridine series with high cytotoxic activity in cisplatin resistant A2780cisR tumor cells and reduced toxicity. J. Inorg. Biochem. 125:26-31.
17. MATESANZ, A.I.; SOUZA, P. 2009. a-N-heterocyclic thiosemicarbazonederivatives as potential antitumor agents: A structure-activity relationships approach. Mini-Rev. Med. Chem. 9:1389-1396.
18. PELOSI, G. 2010. Thiosemicarbazone metal complexes: From structure to activity. Open Crystallograph. J. 3:16-28.
19. PRABHAKARAN, R.; RENUKADEVI, S.V.; KARVEMBU, R.; HUANG, R.; MAUTZ, J.; HUTTNER, G.; SUBASHKUMAR, R.; NATARAJAN, K. 2008. Structural and biological studies of mononuclear palladium(II) complexes containing N-substituted thiosemicarbazones. Eur. J. Med. Chem. 43(2):268-273.
20. PRICE, M.L.P.; JORGENSEN, W.L. 2000. Analysis of Binding Affinities for celecoxib analogues with COX-1 and COX-2 from combined docking and Monte Carlo simulations and insight into the COX-2/COX-1 selectivity. J. Am. Chem. Soc. 122(39):9455-9466.
21. QUIROGA, A.G.; PÉREZ, J.M.; MONTERO, E.I.; MASAGUER, J.R.; ALONSO, C.; NAVARRO-RANNINGER, C. 1998. Palladated and platinated complexes derived from phenylacetaldehyde thiosemicarbazone with cytotoxic activity in cis-DDP resistant tumor cells. Formation of DNA interstrand cross-links by these complexes. Inorg. Biochem. 70:117-123.
22. REBOLLEDO, A.P.; VIEITES, M.; GAMBINO, D.; PIRO, O.E.; CASTELLANO, E.E.; ZANI, C.L.; SOUZA- FAGUNDES, E.M.; TEIXEIRA, L.R.; BATISTA, A.A.; BERALDO, H. 2005. Palladium (II) complexes of 2-benzoylpyridine-derived thiosemicarbazones: spectral characterization, structural studies and cytotoxic activity. J. Inorg. Biochem. 99:698-706.
23. RODRÍGUEZ-ARGÜELLES, M.C.; FERRARI, M.B.; FAVA, G.G.; PELIZZI, C.; TARASCONI, P.; ALBERTINI, R.; DALL'AGLIO, P.P.; LUNGHI, P.; PINELLI, S. 1995.2,6-Diacetylpyridine bis(thiosemicarbazones) zinc complexes: Synthesis, structure, and biological activity. J. Inorg. Biochem. 58(3):157-175.
24. SALMAN, A.K.; MOHAMAD, Y. 2009. Synthesis, spectral studies and in vitro antibacterial activity of steroidal thiosemicarbazone and their palladium (Pd(II)) complexes. Eur. J. Med. Chem. 44(5):2270-2274.
25. TAMAYO, L.V.; BRANDÃO, P.F.; BURGOS, A.E. 2014. Synthesis, characterization, and antimicrobial activity of the ligand 3-methylpyrazole-4-carboxaldehyde thiosemucarbazone and its Pd(II)complex. Phosphorus, Sulfur, and Silicon and the Related Elements. 189:52-59.
26. TWEEDY, B.G. 1964. Plant extracts with metal ions as potential antimicrobial agents. Phytopath. 55:910-917.
Received: 3 February 2014 Accepted: 4 September 2014

Revista U.D.C.A Actualidad & Divulgación Científica por Universidad de Ciencias Aplicadas y Ambientales se distribuye bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.