CIENCIAS EXACTAS Y NATURALES-Artículo Científico
INFLUENCE OF SPATIAL TROPHIC PATTERNS ON THE ZOOPLANKTON COMMUNITY STRUCTURE IN A TROPICAL URBAN RESERVOIR IN BRAZIL
INFLUENCIA ESPACIAL DEL PATRÓN TRÓFICO EN LA ESTRUCTURA DE LA COMUNIDAD ZOOPLANCTÓNICA EN UN EMBALSE TROPICAL URBANO EN BRASIL
Juan Carlos Jaramillo-Londoño1, Simone Paula dos Santos2, Ricardo Motta Pinto-Coelho3
1 Biólogo, Doctor en Biología. Grupo de Investigaciones y Mediciones Ambientales (GEMA), Facultad de Ingenierías, Universidad de Medellín. Apartado Aéreo 1983. Fax (574)3405216 Medellín, Colombia. Corresponding author: jcjaramillo@udem.edu.co
2 Bióloga, M.Sc. Laboratório de Gestão Ambiental de Reservatórios. Instituto de Ciências Biológicas. Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. E-mail: sikapaula@yahoo.com.br
3 Biólogo, D. Rer. Nat. Laboratório de Gestão Ambiental de Reservatórios. Instituto de Ciências Biológicas. Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. E-mail: rpcoelho@globo.com.
Rev. U.D.C.A Act. & Div. Cient. 17(2): 521-528, Julio-Diciembre, 2014
SUMMARY
The effect of nutrients inputs, mainly nitrogen and phosphorus on the structure of the zooplankton community, including diversity, evenness, dominance, and richness, in Pampulha Reservoir in the city of Belo Horizonte, Brazil was evaluated. The samples were taken on 15 September 2009 at 23 sampling stations, covering the entire reservoir. The spatial analysis showed that species richness gradually decreased in those sites with increased nutrients; copepods and rotifers increased in density along this same spatial gradient (mainly Thermocyclops decipiens, Metacyclops mendocinus and Brachionus calyciflorus). Thematic maps describing the horizontal distribution of some variables showed that areas with higher nutrient concentrations were associated with increases in dominance and decreases in diversity and species richness.
Key words: Eutrophication, diversity, nutrients, tropical reservoir, plankton.
RESUMEN
Se evaluó el efecto de la entrada de nutrientes, principalmente nitrógeno y fósforo, en la estructura de la comunidad zooplanctónica, incluida la diversidad, la equidad, la dominancia y la riqueza, en la Represa de Pampulha, en la ciudad de Belo Horizonte, Brasil. Las muestras fueron tomadas el 15 de septiembre de 2009 en 23 estaciones de muestreo, cubriendo completamente el embalse. El análisis espacial mostró que la riqueza de especies disminuyó gradualmente en los sitios con mayor cantidad de nutrientes; los copépodos y rotíferos incrementaron su densidad a lo largo de este mismo gradiente espacial (principalmente Thermocyclops decipiens, Metacyclops mendocinus y Brachionus calyciflorus). Los mapas temáticos que describen la distribución horizontal de algunas variables mostraron que las áreas con concentraciones de nutrientes más altos estuvieron asociados con un incremento en la dominancia y una disminución de la diversidad y la riqueza de especies.
Palabras clave: Eutroficación, diversidad, nutrientes, embalse tropical, plancton.
INTRODUCTION
Among the most notorious effects of pollution and other forms of human impacts on the aquatic ecosystems are the loss of species and the increase in dominance of a few opportunistic organisms (Johnston & Roberts, 2009). Although these approaches are generally accepted, few reports have clearly associated eutrophication with the structural properties of tropical plankton communities on a spatial basis (Tundisi & Matsumura-Tundisi, 2008).
Zooplankton has long been used as an indicator of the trophic state of aquatic ecosystems (Gannon & Stemberger, 1978; Bays & Crisman, 1982; Pejler, 1983; Pinto-Coelho et al. 2005). Nonetheless, variable responses of zooplankton to trophic state are common (Ravera, 1996), perhaps, in part, because zooplankton also respond to other environmental factors such as water chemistry (Pinel-Alloul et al. 1990; Hulot et al. 2000), shoreline disturbances and watershed land use (Stemberger & Lazorchak, 1994; Pinto-Coelho, 1998; Patoine et al. 2000), ambient heterogeneity (Kobayashi, 1997; Hobæk et al. 2002; Hall & Burns, 2003) as well as levels of vertebrate and invertebrate predation (Hanazato & Yasuno, 1989; Walls et al. 1990; Boveri & Quirós, 2007; Manca et al. 2008).
One of the major human-induced changes in aquatic environments is eutrophication, usually caused by external inputs of high concentrations of nutrients (mainly phosphorus and nitrogen) (Paerl, 2005; Yang et al. 2008). Eutrophication has dramatically affected phytoplankton biomass and community in lakes (Anneville & Pelletier, 2000; Dokulil & Teubner, 2005). Eutrophication effects often propagate up to higher trophic levels resulting in changes to the zooplankton community (Ravera, 1980; Lovik & Kjelliberg, 2003; Anneville et al. 2007) such as density, richness, diversity, evenness and dominance (Odum, 1986; Sampaio et al. 2002). Small urban lakes and reservoirs are the aquatic ecosystems that are most affected by cultural eutrophication (Smith, 1998).
Brazil has an immense richness of freshwater ecosystems. Nevertheless, this country is facing a dramatic shift in the water quality of several important systems, caused by a variety of human impacts: dam construction, erosion and silting, eutrophication, contamination with metals and Persistent Organic Pollutants (POPs), habitat fragmentation, introduction of alien species, among others (Pinto-Coelho, 1988; Torres et al. 2007; Tundisi & Matsumura-Tundisi, 2008).
Pampulha Reservoir, located within the city of Belo Horizonte, Brazil is a typical example. Eutrophication of the Pampulha Reservoir was initially detected and characterized by Giani et al. (1988). Since then, several studies have demonstrated the continuous intensification of eutrophic conditions in the reservoir, which has caused recurrent cyanobacteria blooms and outbreaks of aquatic macrophytes (Pinto-Coelho, 1998; Pinto-Coelho & Greco, 1999; Greco & Freitas, 2002; Torres et al. 2007; Pinto-Coelho, 2012). These changes may result in the exclusion of some species such as Bosmina longirostris, B. hagmanni and Scolodiaptomus corderoi and increased population growth of others such as Thermocyclops decipiens, Metacyclops mendocinus and Brachionus calyciflorus (Pinto-Coelho, 2012).
Previous investigations (Pinto-Coelho, 1998; Rietzler et al. 2001; Friese et al. 2010) have demonstrated that most biological and chemical properties of this reservoir are rather homogeneous during the dry season, usually May through late October.
Here, the effect of the nutrient input, mainly nitrogen and phosphorus, on structural attributes of the zooplankton community, including diversity, evenness, dominance, and richness, during a sampling campaign was examined.
MATERIALS AND METHODS
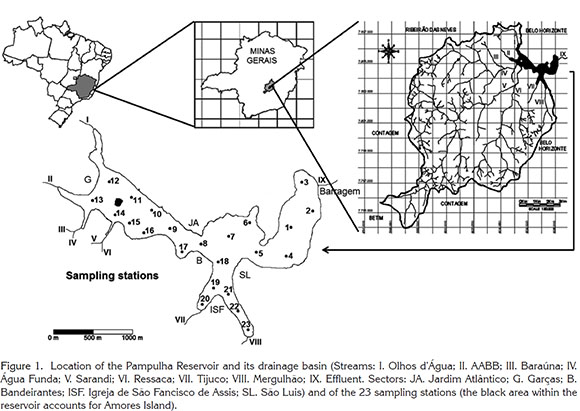
Study area: The Pampulha Reservoir (Figure 1) is located in the northern part (43°56'47''W; 19°55'09''S) of the city of Belo Horizonte, capital of the state of Minas Gerais, Brazil. This is a small artificial lake constructed in 1938, intended as a recreational area and a drinking-water supply. However, the use of the reservoir as drinking water source was interrupted in 1980 because of frequent blooms of blue-green algae. Furthermore, large areas of the lake have been lost by silting in the last four decades. From the original volume of 18 million of m3, actually, the reservoir stores only 9.9 million m3 and the lake area was reduced from 2.1 to 1.9 km2. The maximum depth still remains close to the original of 16 m but the mean depth is now reduced to 5 m (Resck et al. 2008). The architectural complex around the reservoir is a major tourist area for the city, but uncontrolled occupation of the basin has caused extensive deterioration of this water body, mainly by the accelerated eutrophication and decreased depth (Araújo & Pinto-Coelho, 1998).

The Pampulha Reservoir has three different compartments. The first is the shallowest area, the silted area around Amores Island. It is strongly influenced by inputs from the heavily polluted Sarandi and Ressaca rivers and to a lesser extent by Olhos d'Água, AABB, Baraúna, and Água Funda streams. The second area is the deeper middle reservoir, extending from the broader lake surface to the dam. This region has cleaner water and lower densities of algae despite discharges from Tijuco and Mergulhão streams. The third area is an intermediate zone located between Bandeirantes and Jardim Atlântico sectors, with particular conditions that show transitions between the two opposite zones.
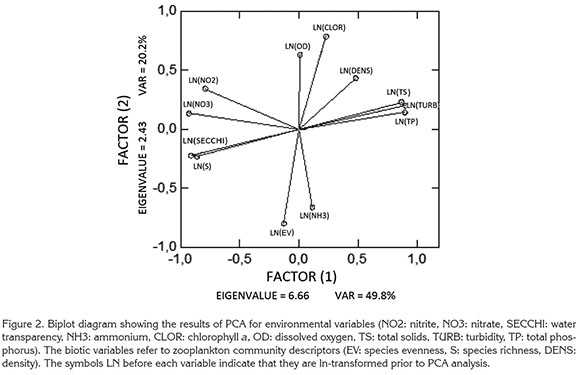
Field and laboratory work: The samples were taken between 10:00 and 17:00 hs on 15 September 2009 at 23 sampling stations, covering the entire reservoir. At each station, depth, water transparency (Secchi disk), chlorophyll a (Fluorimetric Sonde Turner/SCUFA), water temperature, dissolved oxygen and electrical conductivity (Yellow Springs Instruments-YSI multi-parameter probe, model 556) were measured. Subsurface (0.5m depth) water samples were collected in 5L plastic containers for measuring turbidity (DIGIMED model M-3), total solids (gravimetric; Clesceri et al. 1998), total organic nitrogen (semi-micro Kjeldahl; Clesceri et al. 1998) and phosphorus (reaction with ascorbic acid; Clesceri et al. 1998), ammoniun, nitrite and nitrate was measured followed Mackereth et al. (1978) in the laboratory.
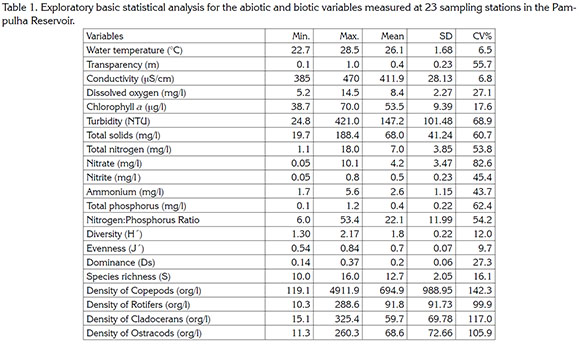
Zooplankton was collected with vertical hauls, from the bottom to the surface, with a plankton net (30 cm diameter and 68 µm mesh). The organisms were preserved with 4% buffered formalin and transported to the Laboratory of Environmental Management of Reservoirs of the Biological Sciences Institute at the Universidade Federal de Minas Gerais. Zooplankton was identified mostly to species level by means of taxonomic keys by Koste (1978), Sendacz & Kubo (1982), Zoppi de Roa et al. (1985), Koste & Shiel (1987), Elmoor-Loureiro (1997), and Fernando (2002). Zooplankton was counted in a Sedgwick-Rafter chamber of 1.0mL. Aliquots of 1.0mL were counted fully to complete at least 400 individuals in each sample, to ensure accuracy not lower than 90% (Edmonson & Winberg, 1971; McCauley, 1984; Pace, 1986). The density was reported in organisms per liter. We used a Leica DMLB microscope at 100x magnification. Using the zooplankton density data, we calculated the community-structure indices: Richness, Diversity (Shannon and Weaver's Index), Dominance (Simpson Index) and Evenness (Pielou Index), with the PAST statistical program. For each variable, we calculated the mean, standard deviation, and Pearson variation coefficient (Table 1). Relationships between variables were established with a Principal Component Analysis (Figure 2). All data variables were ln-transformed (xt=ln(x+1)) prior the analysis. The rotation procedure VARIMAX was used. No resampling was considered. The correlation matrix for extraction was used. Only the first two axis were considered since they host the bulk of total variability (70%). The software SYSTAT version 11 for Windows 7.0 was used (Licence: LGAR-FUNDEP-UFMG).
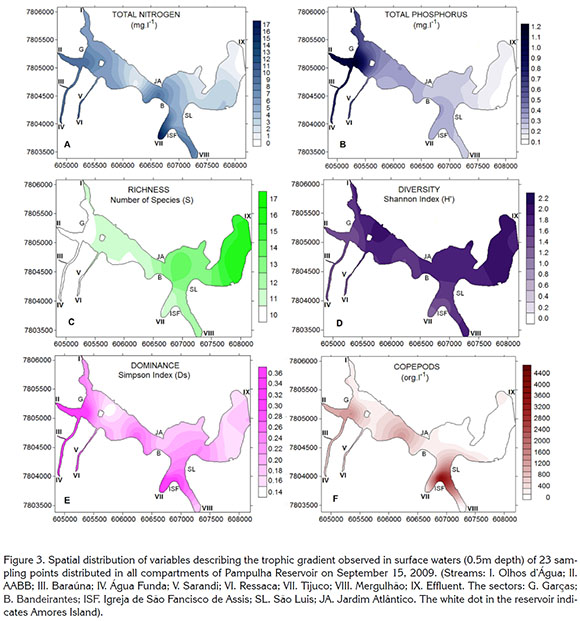
Thematic maps of the horizontal variation of the variables (Figure 3) were obtained using the program Surfer 9.0® (Golden Software Inc.), and the kriging interpolation method was used. The reservoir shoreline was digitized with the program Didger 3.0® (Golden Software Inc.) from a high-resolution image of the Pampulha Reservoir obtained from Google Earth Pro® (Google Inc.). After digitalization, the image was geo-referenced with nine neighboring control points with high-precision coordinates (error <0.05 m) using DGPS GTR-A® (TechGeo Ltda.).
RESULTS AND DISCUSSION
The figure 2 showed the biplot diagram with the results of PCA for environmental variables and the biotic variables refer to zooplankton community descriptors. The first two axis correspond, respectively to 49.8% and 20.2% of total variance.
The PCA factor 1 was able to describe well important zooplankton community species structure descriptors such as total density and total species richness. The PCA showed that the total zooplankton species richness was associated to water transparency and nitrogen forms (nitrates and nitrites) and negative association with the phosphorus concentration, total solids and turbidity. Conversely, total density of zooplankton was associated to variables such as total solids and turbidity. Factor 2 was able to show the expected association between chlorophyll-a and dissolved oxygen was confirmed and this factor showed an association between zooplankton evenness and ammonium.
As shown in table 1 the nitrogen and phosphorus concentrations are typical of a highly eutrophic environment (Tundisi & Matsumura-Tundisi, 2008). The coefficients of variation for the nutrients were above 43% and, overall, the nitrogen concentration was 6 to 53 times higher than the phosphorus concentration.
The highest concentrations of the major nutrients were found in the shallow areas of the reservoir, mainly around Amores Island. This area has higher turbidity and increased biological productivity. Total nitrogen was highest at the mouth of Olhos d'Água and Tijuco streams (Figure 3A), total phosphorus at the mouths of AABB, Baraúna and Água Funda streams (Figure 3B). The highest values for richness of species (Figure 3C) were found in the deeper areas, mainly toward the dam area, near the outflow, in this area the water is clean and has lower densities of algae. Diversity (Figure 3D) was highest near Amores Island and the dam area. The highest values of Dominance (Figure 3E) were found at the mouth of Tijuco stream near the Igreja de São Fancisco de Assis and Copepods (Figure 3F) showed the highest density (15982.2org/L) and were concentrated mainly in the area near the Igreja de São Fancisco de Assis, at the confluence of the Tijuco and Mergulhão streams.
Copepods were represented by two species (Metacyclops mendocinus and Thermocyclops decipiens), cladocerans by four species (Diaphanosoma spinulosum and Bosmina freyi showed the highest densities) and rotifers by seven species and two morphospecies (mainly Brachionus calyciflorus, B. angularis and Trichocerca sp.). One ostracod species was also obnswerved. Adult forms of copepods were most numerous, followed by rotifers, next ostracods and finally cladocerans.
For the community-structure indices, the coefficients of variation were low and generally close to 27%. The densities of all zooplankton groups varied widely, with coefficients of variation of 99% or higher in all cases.
Our short-term survey indicated that horizontal distribution of zooplankton community structure in the Pampulha Reservoir is influenced by the imputs of nutrients, mainly nitrogen and phosphorus, so the species richness decreased along a spatial gradient of nutrients and several highly opportunistic organisms increased in dominance along the same spatial gradient.
Acknowledgments: We thank the biologists Denise Salviano, Denise Pires Fernández and Maíra Campos for logistical support in the fieldwork, and the laboratory technician Cid Antonio Morais for performing the chemical analyses. Con- flict of Interests: The manuscript was prepared and reviewed with the participation of all authors, who declare that there is no conflict of interests that put at risk the validity of the results presented. Financing: This investigation was supported by the educational program ''Curso à distância em Fundamentos em Ecologia e Tópicos em Gestão Ambiental,'' (Conv. 3443-20 FUNDEP-UFMG). The Instituto de Biología and the GAIA group of Universidad de Antioquia awarded a grant to Prof. JC Jaramillo. The M.Sc. student Simone Santos received a grant from MEC-CAPES.
BIBLIOGRAPHY
1. ANNEVILLE, O.; MOLINERO, J.C.; SOUISSI, S.; BALVAY, G.; GERDEAUX, D. 2007. Long-term changes in the copepod community of Lake Geneva. J. Plankton Res. 29:149-159.
2. ANNEVILLE, O.; PELLETIER, J.P. 2000. Recovery of Lake Geneva from eutrophication: quantitative response of phytoplankton. Arch. Hydrobiol. 148:607-624.
3. ARAÚJO, M.A.; PINTO-COELHO, R.M. 1998. Produção e consumo de carbono orgánico na comunidade planctónica da represa da Pampulha, Minas Gerais, Brasil. Rev. Bras. Biol. 58:405-416.
4. BAYS, J.S.; CRISMAN, T.L. 1982. Zooplankton and trophic state relationships in Florida lakes. Can. J. Fish. Aquat. Sci. 39:1813-1819.
5. BOVERI, M.; QUIRÓS, R. 2007. Cascading trophic effects in pampean shallow lakes: results of a mesocosm experiment using two coexisting fish species with different feeding strategies. Hydrobiologia. 584:215-222.
6. CLESCERI, L.; GREENBERG, A.E.; EATON, A.D. 1998. Standard Methods for Examination of Water and Wastewater. 20th Ed. APHA, AWWA, WEF. Baltimore (MD) USA.
7. DOKULIL, M.T.; TEUBNER, K. 2005. Do phytoplankton communities correctly track trophic changes? An assessment using directly measured and paleolimnological data. Freshwater Biol. 50:1594-1604.
8. EDMONSON, W.T.; WINBERG, G.C. 1971. A manual on methods for the assessment of secondary productivity in fresh waters. I. B. P. Handbook n°17. Blackwell Scientific Publications, Oxford, 385p.
9. ELMOOR-LOUREIRO, L. 1997. Manual de identificação de Cladóceros límnicos do Brasil. Editora Universa. Universidad Católica de Brasilia. 156p.
10. FERNANDO, C.H. 2002. A guide to tropical freshwater zooplankton. Identification, ecology and impact on fisheries. Backhuys Publishers, Leiden. The Netherlands. 291p.
11. FRIESE, K.; SCHMIDT, G.; CARVALHO, J.; ARIAS, H.; ZACHMANN, D.W. 2010. Anthropogenic influence on the degradation of an urban lake - The Pampulha reservoir in Belo Horizonte, Minas Gerais, Brazil. Limnologica. 40:114-125.
12. GANNON, J.E.; STEMBERGER, R.S. 1978. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. T. Am. Microsc. Soc. 97:16-35.
13. GIANI, A.; PINTO-COELHO, R.M.; OLIVEIRA, S.J.M.; PELLI, A. 1988. Ciclo sazonal de parámetros fisicoquímicos da água e distribução horizontal de nitrogênio e fósforo no reservatório da Pampulha, Belo Horizonte, MG, Brazil. Ciência e Cultura. 40:69-77.
14. GRECO, M.K.B.; FREITAS. J.R. 2002. On two methods to estimate production of Eichhornia crassipes in the eutrophic Pampulha reservoir (MG, Brazil). Braz. J. Biol. 62:463-471.
15. HALL, C.J.; BURNS, C.W. 2003. Responses of crustacean zooplankton to seasonal and tidal salinity changes in the coastal Lake Waihola, New Zealand. New. Zeal. J. Mar. Fresh. 37: 31-43.
16. HANAZATO, T.; YASUNO, M. 1989. Zooplankton community structure driven by vertebrate and invertebrate predators. Oecologia. 81:450-458.
17 . H OBÆK, A.; MANCA, M.; ANDERSEN, T. 2002. Factor influencing species richness in lacustrine zooplankton. Acta Oecologica. 23:155-163.
18. HULOT, F.D.; LACROIX, G.; LESCHER-MOUTOUÉ, F.; LOREAU, M. 2000. Functional diversity governs ecosystem response to nutrient enrichment. Nature (Lond.). 405:340-344.
19. JOHNSTON, E.L.; ROBERTS, D.A. 2009. Contaminants reduce the richness and evenness of marine communities: A review and meta-analysis. Environ. Pollut. 157:1745-1752.
20. KOBAYASHI, T. 1997. Associations between environmental variables and zooplankton body masses in a regulated Australian river. Mar. Freshwater Res. 48:523-529.
21. KOSTE, W. 1978. Rotatoria: Die Rädertiere Mitteleuropas Überordnung Monogononta. Vol. II. Gebrüder Borntraeger, Berlin. 472p.
22. KOSTE, W.; SHIEL, R.J. 1987. Rotifera from Australian Inland Waters II. Epiphanidae and Brachionidae (Rotifera: Monogonta). Invertebr. Taxon. 7:949-1021.
23. LOVIK, J.E.; KJELLIBERG, G. 2003. Long-term changes of the crustacean zooplankton community in Lake Mjøsa, the largest lake in Norway. J. Limnol. 62:143-150.
24. MACKERETH, F.J.H.; HERON, J; TALLING, J.F. 1978. Water analysis: some revised methods for limnologist. Freshwater Biological Associations Scientific. Washington. 120p.
25. MANCA, M.; VIJVERBERG, J.; POLISHCHUK, L.; VORONOV, D. 2008. Daphnia body size and population dynamics under predation by invertebrate and fish predators in Lago Maggiore: an approach based on contribution analysis. J. Limnol. 67:15-21.
26. McCAULEY, E. 1984. The estimation of the abundance and biomass of zooplankton in samples. In: Downing, J.A.; Rigler, F.H. (Eds.) A manual on methods for the assessment of secondary productivity in freshwaters. p.228-266. 2nd ed. Blackwell Scientific Publication. 500p.
27. ODUM, E.P. 1986. Fundamentos de Ecología. Ed. Interamericana, Mexico. 422p.
28. PACE, M.L. 1986. An empirical analysis of zooplankton community size structure across lake trophic gradients. Limnol. Oceanogr. 31:45-55.
29. PAERL, H.S. 2005. Assessing and managing nutrientenhanced eutrophication in estuarine and coastal waters: Interactive effects of human and climatic perturbations. Ecol. Eng. 25: 40-54.
30. PATOINE, A.; PINEL-ALLOUL, B.; PREPAS, E.; CARIGNAN, R. 2000. Do logging and forest fires influence zooplankton biomass in Canadian Boreal Shield lakes? Can. J. Fish. Aquat. Sci. 57:155-164.
31. PEJLER, B. 1983. Zooplanktonic indicators of trophy and their food. Hydrobiologia. 101:111-114.
32. PINEL-ALLOUL, B.; MÉTHOT, G.; VERREAULT, G.; VIGNEAULT, Y. 1990. Zooplankton species associations in Québec lakes: variation with abiotic factors, including natural and anthropogenic acidification. Can. J. Fish. Aquat. Sci. 47:110-121.
33. PINTO-COELHO, R.M. 2012. Atlas da qualidade de água do Reservatório da Pampulha. Belo Horizonte. Recóleo. 56p.
34. PINTO-COELHO, R.M., PINEL-ALLOUL, B.; MÉTHOT, G.; HAVENS. K.E. 2005. Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: variation with trophic status. Can. J. Fish. Aquat. Sci. 62:348-361.
35. PINTO-COELHO, R.M.; GRECO, M.B. 1999. The contribution of water hyacinth (Eichhornia crassipes) and zooplankton to the internal cycling of phosphorus in the eutrophic Pampulha Reservoir. Hydrobiologia. 411:115-127.
36. PINTO-COELHO, R.M. 1998. Effects of eutrophication on seasonal patterns of mesozooplankton in a tropical reservoir: a 4-year study in Pampulha Lake, Brazil. Freshwater Biol. 40:159-173.
37. RAVERA, O. 1996. Zooplankton and trophic state relationships in temperate lakes. Memorie dell'Istituto Italliano di Idrobiologia. 54:195-212.
38. RAVERA, O. 1980. Effects of eutrophication on zooplankton. Prog. Water Technol. 12:141-159.
39. RESCK, R., BEZERRA-NETO. J.F.; PINTO-COELHO, R.M. 2008. Nova batimetria e avaliação de parâmetros morfométricos da Lagoa da Pampulha (Belo Horizonte, Brasil). Geografias. Revista do Departamento de Geografia-UFMG. 3:24-27.
40. RIETZLER, A.C.; FONSECA, A.L.; LOPES, G.P. 2001. Heavy metals in the tributaries of Pampulha reservoir, Minas Gerais. Braz. J. Biol. 61: 363-370.
41. SAMPAIO, E.V.; ROCHA, O.; MATSUMURA-TUNDISI, T.; TUNDISI, J.G. 2002. Composition and abundance of zooplankton in the limnetic zone of seven reservoirs of the Paranapanema River. Brazil. Braz. J. Biol. 62:525-545.
42. SENDACZ, S.; KUBO, E. 1982. Copepoda (Calanoida e Cylopoida) de reservatorios do estado de São Paulo. Bol. Inst. Pesca. 9:51-89.
43. SMITH, V.H. 1998. Cultural eutrophication of inland, estuarine and coastal waters. In: Pace, M.L.; Groffman, P.M. (Eds.) Successes, limitations and frontiers in ecosystem science. P.7-49. New York. Springer. 499p.
44. STEMBERGER, R.S.; LAZORCHAK, J.M. 1994. Zooplankton assemblage responses to disturbance gradients. Can. J. Fish. Aquat. Sci. 51:2435-2447.
45. TORRES, I.C.; RESCK, R.P.; PINTO-COELHO, R.M. 2007. Mass balance estimation of nitrogen, carbon, phosphorus and total suspended solids in the urban eutrophic, Pampulha reservoir, Brazil. Acta Limnol. Bras. 19:79-91.
46. TUNDISI, J.G.; MATSUMURA-TUNDISI, T. 2008. Limnologia. São Paulo. Oficina de Textos. 632p.
47. WALLS, M.; KORTELAINEN, I.; SARVALA, J. 1990. Prey responses to fish predation in freswater communities. Ann. Zool. Fenn. 27:183-199.
48. YANG, X.; WU, X.; HAO, H.; HE, Z. 2008. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B. 9:197-209.
49. ZOPPI DE ROA, E.; MICHELANGELLI, F.; SEGOVIA, L. 1985. Cladocera (Crustácea, Branchiopoda) de sabanas inundables de Mantecal, Estado Apure, Venezuela. Acta Biol. Venez. 12:43-55.
Received: 31 January 2014 Accepted: 2 September 2014

Revista U.D.C.A Actualidad & Divulgación Científica por Universidad de Ciencias Aplicadas y Ambientales se distribuye bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.